The eye-catching thesis is that in a warming climate, the heat engine that drives the poleward circulation, typified by the Hadley Cell, will work with decreased efficiency, because water vapor will not work as well as a working fluid. Their abstract says:
Incoming and outgoing solar radiation couple with heat exchange at Earth’s surface to drive weather patterns that redistribute heat and moisture around the globe, creating an atmospheric heat engine. Here, we investigate the engine’s work output using thermodynamic diagrams computed from reanalyzed observations and from a climate model simulation with anthropogenic forcing.We show that the work output is always less than that of an equivalent Carnot cycle and that it is constrained by the power necessary to maintain the hydrological cycle. In the climate simulation, the hydrological cycle increases more rapidly than the equivalent Carnot cycle. We conclude that the intensification of the hydrological cycle in warmer climates might limit the heat engine’s ability to generate work.
I think there are some global constraints here, as I said in a comment at ATTP. It's something I've written about here over the years, here, here, and here. Overall, I'm uncertain. I've read the paper, and will continue to try to understand it. But meanwhile, I will give my understanding of Carnot cycles, how Hadley cells emulate one, and what role water vapor plays.
The Carnot Cycle
| Wiki shows this schematic. You could think of a piston like a coffee plunger, with two plates - source hot, sink cold. With the air initially hot, you place it on the hot plate which is at the same temp. It expands and does work, which staying at the constant temperature of the source. After a while, you take it off the plate. It continues expanding and doing work but now cooling. When it cools to the temp of the sink, you put it on that plate, and now start compressing. During this stage, the entropy that was admitted at the source stage is emitted. When it has gone you withdraw, but keep compressing adiabatically until it warms to the temperature of the source. Repeat... |  |
| More formal is this P-V (pressure-volume) diagram from Wiki. The description is here. I'd like to pick up some points. We need the concepts of:
|  |
The heat gained from the source adds directly to the enthalpy. In the first step (1->2), U is constant, so the enthalpy change goes entirely into PV work. In the second, enthalpy doesn't change (adiabatic), so the work done just comes from the change in U. That is the same change in the fourth adiabatic compression stage, so they do no net work. The net work is done by the excess of the first stage over the third.
Hadley Cell
| The Hadley cell is an eddy where warm air rises in the tropics (ITCZ), travels at high altitude supplying heat to support TOA IR emission, then descends in mid-latitudes (subtropical ridge) and returns via the surface trade winds. There are Coriolis effects, but the main heat engine effects can be considered without them.
The subtropical ridge is a region of high pressure, so the mass of air there is high. Where the cell rises, (ITCZ), the surface pressure is lower, so the mass of air is less. However, it is warmer, so the center of mass is actually higher. To think of it as a Carnot cycle, the first thing needed is the reservoir of mechanical energy. Kinetic energy plays some role, but the main one is hydrostatics. The bouyant rise in the tropics carries the air to a high altitude, whence it travels gradually downhill to the descent phase. The potential energy gained carries it through the cooling phase. It is bouyancy that keeps the low pressure at the equaqtor, and negative bouyancy that pushes air into the pressure maximum at the subtropical ridge. The resulting pressure gradient drives the trade wind stage. So next to the Carnot analogy, thinking first of a dry circulation. I've identified four stages. The heat source is the surface, so the step 1, where the working fluid picks up heat, is far from isothermal. The fluid gains internal energy, while still expanding. The expansion contributes by facilitating the gravitational gradient at high altitude. The (adiabatic, approx) rise stage is enhanced by the internal energy thus gained. The expansion does work to maintain a slightly reduced hydrostatic pressure gradient, in turn maintaining the surface low pressure and the pressure gradient that drives the trades. In the third, high altitude stage, heat is lost to the sink (space) by radiation. In the Carnot cycle, mechanical compression maintained the temperature. Here the gradual loss of altitude has the same effect. Potential energy is the reservoir.In the final stage, the cold air compresses and warms. But it was dense air with relatively less far to descend, so the negative bouyancy allows to to recharge the high pressure zone at the subtropical ridge, keeping up the other end of the surface pressure gradient that drives the trade winds. |
Diagrams from Wikipedia 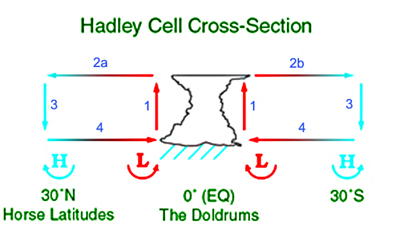 |
Effect of water vapor
That was a dry Hadley Cell. It's generally reckoned that evaporation and latent heat (LH) are powerful extra drivers. This is partly because wv is bouyant, but also because LH increases the heat carrying capacity of the air.LH, if you count it as internal energy, is transferred to the air in addition to sensible heat at the trade wind stage, more especially at the later, warmer stage. But the necessary evaporation takes heat from something. Partly sea water, partly air. The cooled air may be further heated by the air-sea temp differential thus created, but still, there is probably less sensible heat taken up than in the dry case.
As I understand it, Lalibertie et al are saying that in fact you can't count the latent heat until it does actually condense. And as global warming proceeds, LH is a greater proportion of internal energy, and this is a limitation.
But obviously water vapor does condense somewhere along the cycle. And most of it will have done so before the end of the bouyant rise. In doing so, it warms (or slows cooling) of the rising air, and allows it to rise to a greater height than it otherwise would have. Or put another way, it helps maintain the surface low pressure, and thus the surface pressure gradient that drives the trade winds. So the fact that the condensation occurs later does not diminish the work available to the cycle.












typified by the Hadley Cell, with work with decreased efficiency
ReplyDeleteI think you mean "will work".
Indeed so, thanks.
DeleteVery interesting. I think I've kind of got what you're suggesting and it makes sense. I may have to read it a few more times though.
ReplyDeleteNick,
ReplyDelete"As I understand it, Lalibertie et al are saying that in fact you can't count the latent heat until it does actually condense. And as global warming proceeds, LH is a greater proportion of internal energy, and this is a limitation."
When the authors step through the Carnot cycle (in the supplemental) they say: 1. The first step is to add a small amount of liquid water to the air parcel isothermally at input temperature T in and isobarically at input pressure Pin.
Which has been throwing me, and one place I'm fairly sure I flubbed it at ATTP's -- I wasn't thinking about the additional energy from anthro forcings which ARE available to work irrespective of the condensation restraint. So more LH to move to the cool reservoirs where it can condense out, with less relative free energy to get it there. I think from there the argument goes it's not less work being done overall, but less work relative to total energy in the system.
I think from there the argument goes it's not less work being done overall, but less work relative to total energy in the system.
DeleteThat was kind of what I had thought, too, but I think the Laliberte et al. paper is actually arguing that it is less work overall, not just less work relative to the total energy in the system.
Crap. W = Qtotal - Qmoist. Mutter mutter ....
DeleteMore droughts, but also more extreme precipitation events... Does that take less work?
ReplyDeleteNick: But obviously water vapor does condense somewhere along the cycle. And most of it will have done so before the end of the bouyant rise. In doing so, it warms (or slows cooling) of the rising air, and allows it to rise to a greater height than it otherwise would have.
ReplyDeleteThat additional warming is associated with the tropical hot spot people are discussing. But the effect you described here is exactly the mechanism that drives the the entrained warm moist to higher altitudes in large thunderstorms. So I think this observation is on the money.
I haven't read the paper in question, but it is clearly an error not to consider the effect of phase transitions here. It's well understood in any case that the air in the Hadley cell circulation loses its moisture as it rises from the ITCZ, and the dry descending air is responsible for the subtropical "dry belt"
Carrick,
DeleteThe paper is thinking of phase transitions - especially evaporation. It just doesn't seem to take a whole cycle view. I might have missed it, though.
Nick,
ReplyDeletethanks for this, very clear and I'm now in danger of thinking I understand the basics at least.
Hurricanes are also written of as being heat engines. From my reading on that, and this post, I think the main differences are:
-heat source- sensible heat from a hot ocean for hurricanes, insolation for hadley cells.
- rate of heating of cold returning air. Much higher for hurricanes, where the source is practically unlimited, but limited by solar flux for hadley cells.
Does that sound at all right?
VTG,
DeleteYes. I think hurricanes are a bit harder to see as a closed cycle, because the descent stage is more diffuse. It's an interesting point you make about heat source - I had been just thinking of the tradewinds picking up heat from the surface, but yes, they would also get much heat from increasing insolation (warming clouds etc).
Nick,
DeleteI was making a slightly different point. That for hurricanes the heat release can presumably massively exceed mean insolation as it depends on the sensible heat available from a hot ocean, the rate of release of which is limited by heat & mass transfer at the surface. Which is very high due to the strength of the winds.
Whereas for Hadley cells, the heat release from the surface can't exceed the mean insolation at the surface (otherwise the tropics would cool over time)
In other words, hurricanes are a transient phenomenon, whereas the Hadley cells operate continuously.
Nick,
ReplyDeleteWhat you present is basically in agreement of my thinking including this comment at aTTP.
Concerning the role of evaporation and condensation (in particular the latter) higher surface temperature leads to more evaporation. The extra water is moved to higher altitude. In that the gravitational potential energy of the water molecules increases, but after condensation that typically stops. The molecules remain for a while as droplets (or ice) in the cloud. At that stage they cause a drag for the uplift of air. Thus they dissipate part of the work the heat engine of the Hadley cell could otherwise produce. Further on, the condensed water starts to precipitate. In that it falls down, and all it's gravitational potential energy is lost - again a form of dissipation. But not only that, but the falling droplets cause a continuous drag for the rising air through which they fall adding to the dissipation.
Entropy generation has taken place due to the moisture even before all of that. The evaporation takes place essentially isentropically, when we consider it at the small micro level within a few mean free paths from the surface, but the relative humidity is usually smaller in much of the atmospheric boundary layer. Mixing the moist air from the immediate neighborhood of the surface to the less moist ABL means entropy production. The amount of that can be calculated also by assuming that the evaporation takes directly place to the lower typical moisture of the boundary layer.
The large scale circulation of the Hadley cell is, however, only part of the story. Much of the free energy is lost in more local weather phenomena, and humidity plays a major role in that as well.